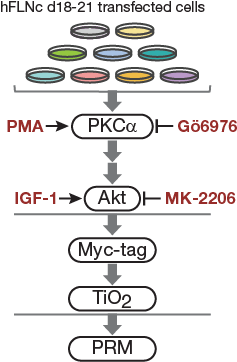
Fig4C.
Cell-based kinase assay. PKC activator PMA, PKCa inhibitor Gö6976 and Akt activator IGF-1 and inhibitor MK-2206 were used to activate or block signaling pathways in C2 cells. For targeted MS analysis, phosphopeptides were enriched by Myc-tag and TiO2-based enrichment.

Figure 4D.
Immunoblot analysis of PKCα and Akt activities in C2 cells following pharmacologic interventions as indicated in 4C. Pan- and phospho-specific antibodies were used to detect total protein amounts and phosphoisoforms. GAPDH was used as loading control.
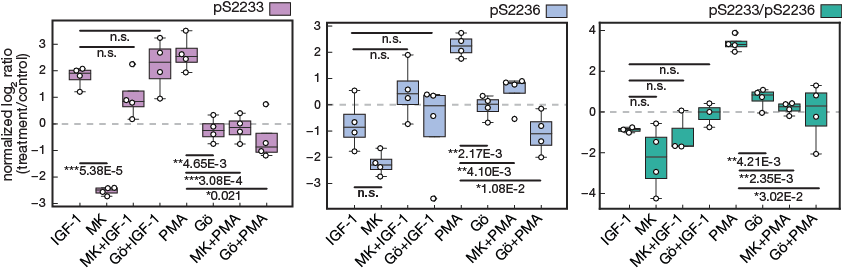
Figure 4e.
Targeted MS data of hFLNc phosphopeptides comprising the phosphorylation sites pS2233, pS2233/pS2236 and pS2236. MS data were quantified using Skyline and normalized to an internal phosphopeptide standard and the mock-treated control (DMSO). Intensities of phosphopeptides distinctive for a specific phosphorylation site in hFLNc d18-21 WT were added up per experiment and represented as normalized mean log2 ratio (treatment/control) ± SEM (n=4; *, p ≤ 0.05; **, p ≤ 0.01)
This data is available under the CC BY 4.0 license.