Site-resolved, large-scale analysis of the yeast redoxome |
2024-04-19 |
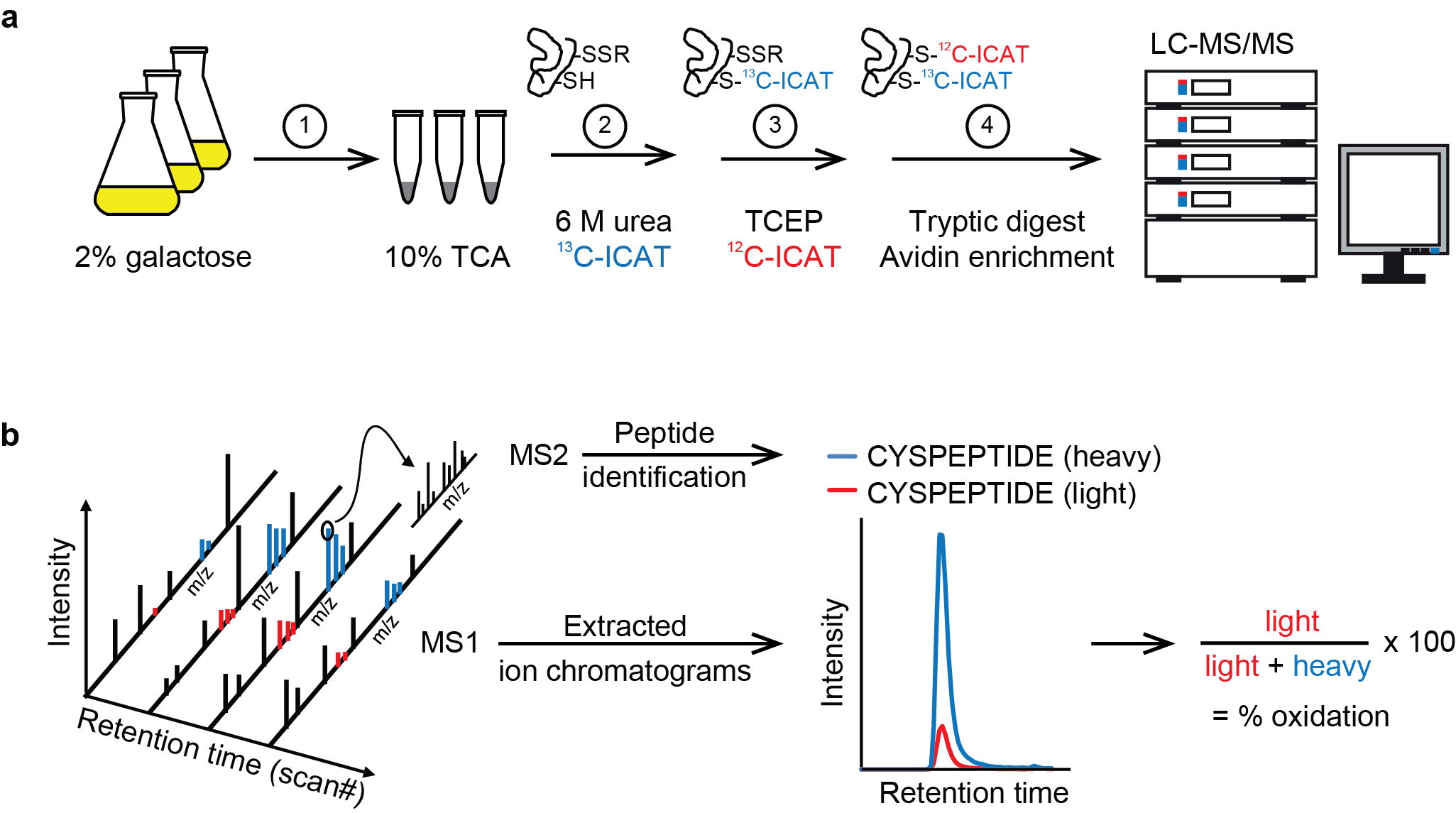
Site-resolved, large-scale analysis of the yeast redoxome. Yeast cells grown in 2% galactose medium were immediately frozen in 10% TCA (1). Extracted proteins were denatured in 6 M urea in the presence of heavy ICAT (13C-ICAT) to label free thiol groups (2). Reversibly oxidized cysteine residues were reduced by TCEP and labeled by light ICAT (12C-ICAT) (3). Proteins were digested with trypsin and ICAT-labeled peptides were enriched by streptavidin affinity chromatography (4). Following cleavage of the biotin tag, peptides were analyzed in duplicate by LC-MS/MS. (b) For the accurate determination of the redox state of cysteine residues, peptides were identified based on fragment ions observed in MS2 spectra using MaxQuant (v.1.4.1.2) and then quantified by extracting MS1 ion chromatograms using Skyline (v.2.5.0). Following integration of peak areas of heavy and light peptide variants, the proportion of reversibly oxidized cysteine residues (% oxidation) was calculated.